Answer : Temperature and kinetic energy have a direct relationship.
Explanation :
The relationship between the kinetic energy of a gas particle and the temperature is,
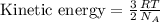
where,
R = Gas constant
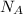 = Avogadro's number
= Avogadro's number
T = temperature
From this we conclude that the kinetic energy of a gas molecule is directly proportional to the the temperature of a gas molecule. That means there is a direct relationship between the kinetic energy and the temperature.
Hence, the temperature and kinetic energy have a direct relationship.