Answer:
0.16M
Explanations:
The formula for calculating the molarity of a substance is expressed as;
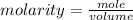
Given the following parameters
• mole of HCl = 0.24 mol
,
• volume of the solution = 1.5L
Substitute the given parameters into the formula to have:
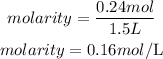
Therefore the molarity of the solution is 0.16M