ANSWER:
Step-by-step explanation
Given that:
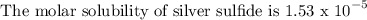
To find the solubility constant product for the compound, follow the steps below
Step 1: Write the ionic equation for silver sulfite
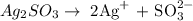
From the above reaction, the silver sulfite split into silver ion and sulfite ion
![undefined]()