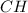Answer:
Step-by-step explanation:
Given the empirical formula and the molar mass of the compound, we want to get its molecular formula
The molecular formula can be obtained from the empirical formula by dividing the molar mass of the molecular formula by the molar mass of the empirical formula, to get a number, let us call it n
We will use this n to multiply the subscript of the atoms in the emirical forrmula so as to get the molecular formula
The mass of the formula CH is (13 g/mol which is obtained by adding the atomic mass unnit of carbon which is 12 amu and the atomic mass unit of hydrogen, which is 1 amu)
By dividing, we get the value 1 (since the mass of the empirical formula is same as the mass of the molecular formula)
This means the empirical formula is the same as the molecular formula