Answer: there are 127.5g of N2 in 4.55 moles of this molecule
Step-by-step explanation:
The question requires us to calculate the mass that corresponds to 4.55 moles of nitrogen (N2).
To solve this problem, we can use the molar mass of N2, which correlates the mass of the molecule with one mol of it.
The atomic mass of nitrogen is 14.01 u. Thus, the molar mass of nitrogen gas (N2) is:
molar mass N2 = 2 * 14.01 = 28.02 g/mol
Now, we know that there are 28.02g of N2 in 1 mol of this gas, thus we can write:
1 mol N2 ------------------- 28.02g N2
4.55 mol N2 -------------- x
Solving for x, we'll have:
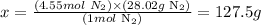
Therefore, there are 127.5g of N2 in 4.55 moles of this molecule.