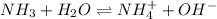Step-by-step explanation:
- If an acid is strong in nature then upon dissolving in water it will completely dissociate into ions.
For example, HCl is a strong acid and when we dissolve it in water then it will dissociate into hydrogen (
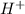 ) and chlorine (
) and chlorine (
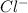 ) ions.
) ions.
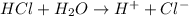
- And, if an acid is weak in nature then it will partially dissociate into ions.
For example, acetic acid is a weak acid and it will dissociate as follows when dissolved in water.
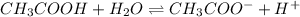
- Similarly, if a base is strong in nature then it will completely dissociate into ions.
For example, NaOH is a strong base and when it is added to water it will dissociate as follows.
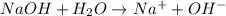
- And, when a base is weak in nature then it will partially dissociate upon dissolution in water.
For example, ammonia is a weak base and it will dissociate as follows when added to water.