Answer:
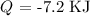
Step-by-step explanation:
Here, we want to calculate the heat energy lost by the water
Mathematically, we can get that using the following mathematical relation:
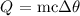
where Q is the amount of heat
C is the specific heat capacity of water
delta theta is the temperature change which is the difference between the final and initial temperature
The mass of 53 mL of water is 0.0534 kg which is 53.4 g
Substituting the values, we have it that:
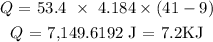
Since it was cooled down, heat is lost which indicates a negative value