Answer: The electronic configuration of zinc is given below.
Step-by-step explanation:
Electronic configuration is the representation of all the electrons that are present around the nucleus of an atom.
Number of electrons are determined by the atomic number of an element.
We need to write the electronic configuration of zinc element.
Atomic number of zinc = 30 = Number of electrons
Electronic configuration of zinc element is:
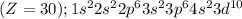
Hence, the electronic configuration of zinc is given above.