Answer: The correct answer is'when adding a few drops of base causes a large increase in pH'.
Step-by-step explanation:
When an acid reacts with base neutralization reaction occurs which means that H^+ ions from the acid and OH^- ion from the base combine together to form neutral molecule of water.
- If the concentration of
 is slightly higher than the
is slightly higher than the
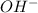 concentration then the pH of the solution will become lower than 7.
concentration then the pH of the solution will become lower than 7.
- If the concentration of
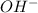 is slightly higher than the
is slightly higher than the
 concentration then the pH of the solution will become higher than 7.
concentration then the pH of the solution will become higher than 7. - If concentration of both
 and
and
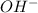 are equal then the pH of the solution will be 7. That is neutral pH.
are equal then the pH of the solution will be 7. That is neutral pH.
During the titration , while adding base to an acid. Addition of base must be stopped when large increase in value of pH of an acid is observed on a pH meter.