Answer: The electron configuration of nitrogen (N) is 1s22s22p3.
Step-by-step explanation:
When electrons of an atom are distributed in a molecular orbital then this distribution is known as electronic configuration.
Atomic number of nitrogen is 7 and its electronic distribution is 2, 5.
Whereas the electronic configuration of nitrogen is as follows.
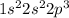
Thus, we can conclude that out of the given options only option (A) depicts correct electron configuration of nitrogen (N).