Step-by-step explanation:
It is given that volume is 375 mL and
 is 37.5 degree celsius.
is 37.5 degree celsius.
First, we will calculate the mass of sample of water as it is known that density of water is 1
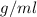 .
.
Hence, Density =
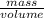
1
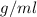 =
=
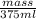
mass = 375 g
Therefore, formula to calculate heat loss will be as follows.
q = mC

where q = heat lost or absorbed
m = mass
C = specific heat of water = 4.186 J/g
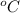
 = change in temperature
= change in temperature
Hence, we will calculate value of heat lost as follows.
q = mC

= 375 g × 4.186 J/g
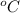 × ( 0 - 37.5) degree celsius
× ( 0 - 37.5) degree celsius
= - 58865.62 joules
Thus, we can conclude that heat lost is -58865.62 joules.