Answer:
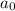 =2,
=2,
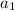 =25,
=25,
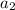 =16,
=16,
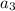 =18
=18
Step-by-step explanation:This is an oxidation reaction in which alkane
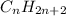 is getting oxidised to give carbon dioxide and water.
is getting oxidised to give carbon dioxide and water.
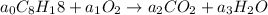
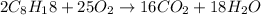
According to the law of conservation of mass, the number of atoms on product side must be equal to the number of atoms on reactant side so s the mass remains conserved.
Thus
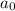 =2
=2
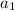 =25
=25
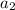 =16
=16
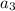 =18
=18