Answer: Molecular mass of ethylene glycol is 62 g/mol and molality of solution will be 9.39 mol/kg.
Step-by-step explanation:
- To calculate the molar mass of ethylene glycol, we add the mass of each element multiplied with their individual atoms.
Mass of oxygen atom = 16 g/mol
Mass of hydrogen atom = 1 g/mol
Mass of carbon atom = 12 g/mol
Mass of ethylene glycol =
![[(2* 12)+(6* 1)+(2* 16)]=62g/mol](https://img.qammunity.org/2018/formulas/chemistry/high-school/92oc8cn9t6ge1vk0p8uiw50gnuxx8njbaa.png)
- Molality of the solution is defined as the ratio of moles of solute to the mass of solvent present in kilograms.
Mathematically,
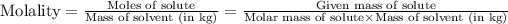
Water is the solute and ethylene glycol is the solvent in this case.
We are given:
Mass of solute (water) = 1.2 kg = 1200 g (Conversion factor: 1 kg = 1000 g)
Molar mass of solute (water) = 18 g/mol
Mass of solvent (ethylene glycol) = 7.1 kg
Putting values in above equation, we get:
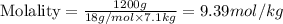
Hence, molecular mass of ethylene glycol is 62 g/mol and molality of solution will be 9.39 mol/kg.