Answer
The actual yield of iron in moles = 0.313 mol
The theoretical yield of iron in moles = 0.672 mol
The percent yield = 46.58%
Step-by-step explanation
Given:
Moles of Fe₂O₃ that react = 0.336 mol
Mass of Fe produced = 17.5 g
Equation: Fe₂O₃ + 3C → 2Fe + 3CO
What to find:
i. The actual yield of iron in moles.
ii. The theoretical yield of iron in moles.
iii. The percent yield.
Step-by-step solution:
i. The actual yield of iron in moles.
From the given information; the actual yield of Fe in grams is 17.5 g.
So, the actual yield in moles can be determined by converting grams to mole using the mole formula.
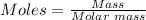
Using the periodic table, the molar mass of Fe = 55.845 g/mol
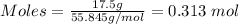
Therefore, the actual yield of iron in moles is 0.313 mol.
ii. The theoretical yield of iron in moles.
Using the mole ratio Fe₂O₃ and Fe, and the given moles of Fe₂O₃; theoretical yield of Fe in moles is calculated as follows:
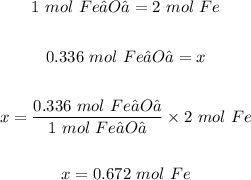
Thus, the theoretical yield of iron in moles is 0.672 mol.
iii. The percent yield.
The percent yield is calculated using the formula:
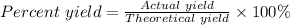
Putting the actual yield = 0.313 mol and the theoretical yield = 0.672 mol into the formula, we have the percent yield to be equal:
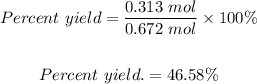
The percent yield is 46.58%