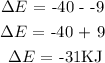ANSWER
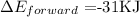
Step-by-step explanation
Given that;
The energy on the product side = -40KJ
The energy on the reactant side = - 9KJ
To find the change in energy, follow the steps below
From the energy diagram provided, The energy level on the product side is higher than the energy level on the reactant side
Step 1: Write the change in energy formula
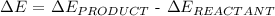
Step 2: Substitute the given data into the above formula