Answer: The percent ionization of HA is 0.26 %
Step-by-step explanation:
We are given:
Molarity of solution = 0.10 M
Let us assume that
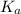 of the given acid is
of the given acid is
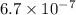
The chemical equation for the ionization of HA follows:
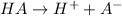
Initial: 0.1
At eqllm: (0.1-x) x x
The expression of
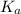 for above equation follows:
for above equation follows:
![K_a=([H^+][A^-])/([HA])](https://img.qammunity.org/2018/formulas/chemistry/high-school/sbyfrky03tp12nb6zjk5fz8u7ve2g4w8q6.png)
We are given:
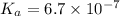
Putting values in above equation, we get:
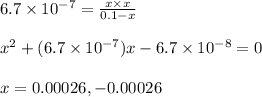
Neglecting the negative value of 'x'.
To calculate the percent ionization, we use the equation:
![\%\text{ ionization}=([H^+]_(eq))/([HA]_i)* 100](https://img.qammunity.org/2018/formulas/chemistry/high-school/rv217gtjuu8pyvdstbl3f2htzv0v6b7zal.png)
![[H^+]_(eq)=x=0.00026M](https://img.qammunity.org/2018/formulas/chemistry/high-school/hlyxxatsja6bxtxq14m1y5wvpt4oq79a44.png)
![[HA]_i=0.1M](https://img.qammunity.org/2018/formulas/chemistry/high-school/dizgcmahpwyh92qc6kgjzp5j5snuomcyu5.png)
Putting values in above equation, we get:
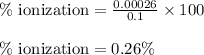
Hence, the percent ionization of HA is 0.26 %