Answer:
The volume of emerald is 276.9 cubic centimeters.
Explanation:
For this we have to use the density equation:
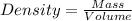
In this case we have a value of density and mass, replacing in the equation we can find the volume value:
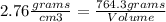
Clearing Volume:
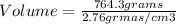
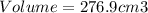
The volume of emerald is 276.9 cubic centimeters.