Explanations:
A chemical equation is known to be balanced if the number of moles of element at the reactant is equal to the number of moles of element at the product.
For the reaction
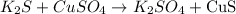
The reaction occurs since there is the production of new products.
For the reaction
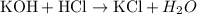
The reaction produces potassium chloride and water. This is a double displacement reaction since the reactants give rise to the formation of a new product (reaction occurs)
For the reaction;
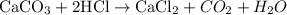
A reaction occurs since it is an acid-base reaction giving rise to the formation of salt and water.
For the reaction
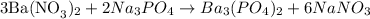
This is also a double displacement reaction since the reactant gave rise to the formation of two new compounds. Hence a reaction occurs.