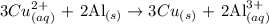Given:
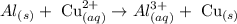
Required: To balance the equation.
Solution
Step 1: Write the half reactions.
The aluminum is oxidized since the oxidation state increases from zero to plus three. The copper is reduced since the oxidation state decreases from 2+ to zero. The half reactions are as follows.
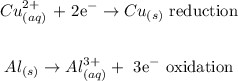
Step 2: Multiply by an appropriate factor to balance the equation
Multiply the reduction reaction by 3
Multiply oxidation reaction by 2
Add the half reactions to get the net ionic equation:
Answer