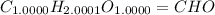The question requires us to find the empirical formula of a compound that contains 40.002% carbon, 6.713% hydrogen, and 53.285% oxygen by mass.
To find the empirical formula of a compound, we need to go through the following steps:
1) Use the percent composition given and find the mass of each element in a 100g sample of the compound;
2) Divide the mass obtained for each element by its respective molar mass in order to find the number of moles of each element;
3) Divide the values obtained in step 2 by the smallest value obtained in order to find a whole number ratio;
4) Write down the formula with the coefficients found in step 3, and, if necessary, adjust the coefficients mathematically to obtain all integer numbers.
Next, we'll go through the steps above to solve the problem:
1) Considering a sample of 100g of the compound, we would have:
40.002% of C = 40.002g of C
6.713% of H = 6.713g of H
53.285% of O = 53.285g of O
2) Now, we must find the number of moles that correspond to each mass obtained in step 1. We'll adopt the following atomic masses:
atomic mass of C = 12.011 u
atomic mass of H = 1.0078 u
atomic mass of O = 15.999 u
We'll find the number of moles by dividing the mass of each element by its respective atomic mass (in g/mol):
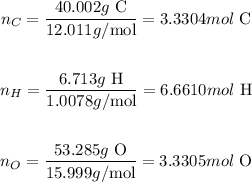
Thus, the number of moles of C, H and O are 3.3304, 6.6610 and 3.3305, respectively.
3) Now, we must divide the values obtained in step 2 by the smallest value obtained (3.3304) to find coefficients for our empirical formula:
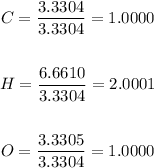
Therefore, the coefficients for C, H and O are 1.0000, 2.0001 and 1.0000, respectively.
4) We can write the empirical formula using the coefficients found in the previous step: