Answer
6.3 moles of mercury are produced.
Step-by-step explanation
Given:
The moles of mercury oxide that decompose = 6.3 mol
Equation: 2HgO(s) ⇨ 2Hg(l) + O2(g)
What to find:
The moles of mercury produced by the decomposition of 6.3 moles of mercury oxide.
Solution:
According to the balanced equation for the reaction;
2 moles of HgO produced 2 moles of Hg
6.3 moles of HgO will produce x moles of Hg
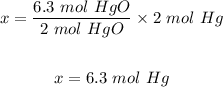
Hence, 6.3 moles of mercury are produced by the decomposition of 6.3 moles of mercury oxide.