We have the balanced equation. To determine how many moles of A are required we must review the stoichiometric coefficients of the reaction. The stoichiometric coefficients are the numbers that come before the molecule.
The stoichiometric coefficient of A is equal to 4 and that of B is 5, therefore the ratio of A to B is equal to 4/5.
If we have 1 mole of B, the moles of A needed are:
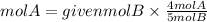
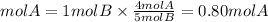
Answer: Second option. Are needed 0.80 mol of B