Answer:
71 mg
Explanation:
nickel-63 has half-life of about 96 years. after 336 years
Initial value is 800 mg sample
half life of nickel-63 is 96 years
time given is 336 years
we use half life formula to get the71 mg amount of nickel remains
Final value = initial value (1/2)^(Time /t(half life ))
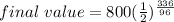
now simplify it
336/ 96 = 7/2
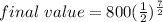
= 70.7106
so answer is