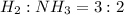Answer: The mole ratio of
Step-by-step explanation:
Mole ratio is defined as the ratio of moles of the chemical species present in a chemical reaction.
In a chemical reaction, it is the ratio of the stoichiometric coefficients.
For the given chemical reaction:
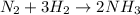
Moles of hydrogen gas = 3
Moles of nitrogen gas = 1
Moles of ammonia gas = 2
So, the mole ratio of hydrogen to ammonia = 3 : 2
Hence, the mole ratio of