Answer : The gram formula mass of
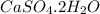 is, 172 g/mole
is, 172 g/mole
Explanation :
As we know that,
Molar mass of calcium (Ca) = 40 g/mole
Molar mass of sulfur (S) = 32 g/mole
Molar mass of oxygen (O) = 16 g/mole
Molar mass of hydrogen (H) = 1 g/mole
Now we have to calculate the gram formula mass of
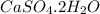
In
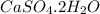 , there are 1 calcium atom, 1 sulfur atom, 6 oxygen atom and 4 hydrogen atom.
, there are 1 calcium atom, 1 sulfur atom, 6 oxygen atom and 4 hydrogen atom.
The gram formula mass of
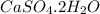 =
=
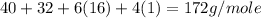
Therefore, the gram formula mass of
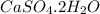 is, 172 g/mole
is, 172 g/mole