Answer:
3.2 calories
Step-by-step explanation:
The state of aggregation of the glass does not change, as its temperature increases, it is a sensible heat, because it depends on its temperature.
The sensible heat formula is
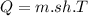
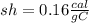
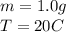
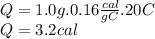
1.0 gram of glass will rise 20.0°C when 3.2 calories of heat are applied