ANSWER
The percent composition of mercury in the sample is 93%
STEP-BY-STEP EXPLANATION:
From the question provided, we can see that the sample is a mixture of mercury and oxygen
Given information
• Mass of sample = 50g
,
• Mass of pure mercury = 46.5g
Since the sample is a mixture of mercury and oxygen. We are not given the mass of oxygen in grams that are present in the sample. So, we need to find the mass of oxygen first
Sample = mercury + oxygen
Let the mass of oxygen be x
50 = 46.5 + x
Subtract 46.5 from both sides
50 - 46.5 = 46.6 + x - 46.5
x = 3.5g
From the above calculation, the mass of oxygen in grams in the sample is 3.5g
The next step is to find the % of mercury in the sample using the below formula
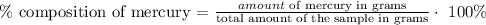

The percent composition of mercury in the sample is 93%