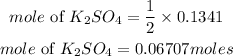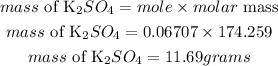Answer:
Explanations:
Given the chemical reaction
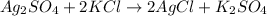
Given the following
Mass of Ag2SO4 = 30grams
Mass of KCl = 10grams
Determine the moles of the reactants
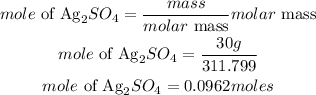

B) B) Since the 1 moles of KCl is lower than the moles of Ag2SO4, hence KCl willl be the limiting reactant.
A) A) According to stoichiometry, 2 moles of KCl produces 2 moles of AgCl, the mass of AgCl produced will be given as;
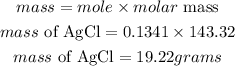
C) According to stoichiometry, 2 moles of KCl produces 1 moles of K2SO4, the mole of K2SO4 produced is;