Answer : (A) greater than
Explanation :
we know that every objects have some energy. It can be kinetic, potential, vibration or rotation. According to the first law of thermodynamics, energy can neither be created nor destroyed but it converts into one form to another.
Kinetic energy is related with the motion of atoms or molecules.
In the chemical reaction, the reactant molecules converted into product molecules when there will be a effective collision between the reactant molecules and for the effective collision of the molecules, the molecules requires kinetic energy.
Average kinetic energy formula is,
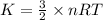
where,
K = Average kinetic energy
n = Number of moles
R = Gas constant
T = temperature
According to the formula, the average kinetic energy is directly proportional to the temperature. Average kinetic energy depends on the temperature. This means that when the temperature of the molecule is high then the average kinetic energy of the molecule is high.
When heat flow from system (1) to another system (2) this means that the temperature will be more in system (1) as compared to system (2). And as we know that the kinetic energy is depend on temperature, the average kinetic energy is more in system (1) as compared to system (2).
when the heat transfer from sample A to sample B, the average kinetic energy of sample A is greater than the average kinetic energy of the sample B.