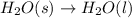Answer: 2. Image A shows a chemical reaction, and image B shows a physical change.
Explanation: A. Chemical change is defined as a change in which rearrangement of atoms take place and leads to formation of new substances.
Roasting of marshmallows will take place in the presence of oxygen and thus the marshmallows will be oxidized.
B. Physical change is defined as a change which leads to change in shape, size or state of the substance changes. No new substance is formed.
Melting of ice only changes the phase of
 from solid to liquid form and thus is a physical change.
from solid to liquid form and thus is a physical change.