We have an initial concentration of a stock solution that has molarity equal to 1M. In a previous question, the stock solution was diluted 10 times, this solution was called solution A. The concentration of solution A found was equal to 0.1M.
Now, we are asked to find the concentration of solution B that starts from solution A.
The initial concentration C1 is the concentration of solution A, equal to 0.1M. The initial volume (V1) is 10mL, and the final volume (V2) is 100mL. We will apply the dilution equation that says:
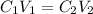
We clear C2 and replace the known data:
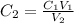
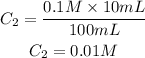
Answer: The molarity of solution B is 0.01 M