Answer: Option (C) is the correct answer.
Step-by-step explanation:
A compound formed by sharing of electrons between its participating atoms is known as a covalent compound.
For example,
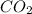 is a covalent compound.
is a covalent compound.
Most covalent compounds are non-polar in nature and they do not dissociate into ions when dissolved in a polar solvent.
Whereas a compound which are formed by transfer of electrons between its participating atoms is known as an ionic compound. These compounds are polar in nature and dissociate into ions when dissolved in a polar solvent like water.
Therefore, we can conclude that it is true regarding covalent substances placed in water that covalent substances don't dissociate into charged particles.