We can use the following formula relating change in volume, change in temperature, and volume coefficient of expansion:
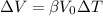
where:
ΔV: change in volume
ΔT: change in temperature
V0: initial volume
Now, the copper radiator also expands when heated along with the fluid, so we need to take that into account. The total change in volume is equal to the sum of the changes in volume of the copper and the fluid.
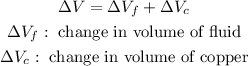
So therefore,
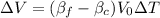
From reference tables and the information given in the problem, we can list the variables we know:
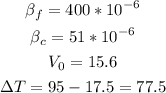
With these variables, we can solve for ΔV.

Finally,
ΔV = 0.421941 L