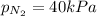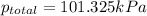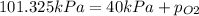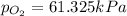Answer: The partial pressure of
 is 61.325 kPa.
is 61.325 kPa.
Explanation:
STP is the abbreviation for Standard Temperature and Pressure. The standard temperature is 273 K and the standard pressure is 1 atm pressure.
1 atm = 101.325 kPa
According to Dalton's law, the total pressure of a mixture of gases is the sum of individual pressures exerted by the constituent gases.
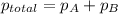
Thus
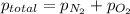
Given: