Molarity is a way of expressing the concentration of a solute in a solution, it is expressed with the term M and can be described by the following equation:
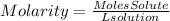
Moles of solute = 0.450 mol
Lsolution= Liters of water+Liters of NaCl
We will assume in this case that the volume of NaCl is negligible compared to the volume of water. Therefore, the volume of the solution will be 145.0 mL or 0.1450L
We replace the known data:
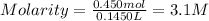
Answer: The molarity of the NaCl solution is 3.1M