Answer: Wavelength which represented photons with :
a) Higher energy is 372.1 nm
b) Longer wavelength is 376.4 nm
c) Higher frequency is 372.1 nm
Step-by-step explanation:
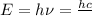 (Planck's equation)
(Planck's equation)
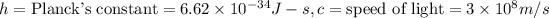
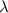 = wavelength of the photon with energy E in meters.
= wavelength of the photon with energy E in meters.
 = frequency of the photon with energy E in hertz.
= frequency of the photon with energy E in hertz.
For first spectral line:
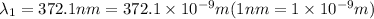
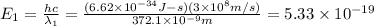 joules
joules
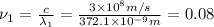 Hertz
Hertz
For second spectral line:
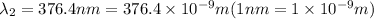
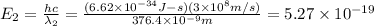 joules
joules
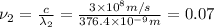 Hertz
Hertz