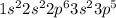Answer: The electronic configuration of Chlorine is
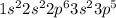
Step-by-step explanation: Electronic configuration is the distribution of electrons of an atom of molecule in atomic or molecular orbitals respectively.
Chlorine is an element present in group 17 of the periodic table. It's atomic number is 17.
Number of electrons = Atomic number
Number of electrons in Cl atom = 17
Electronic configuration of Cl will be