Assuming all the HCl presend in the 24.06mL reacted, we can follow the steps:
1 - Use the concentration and the volume to calculate the number of moles of HCl that reacted
2 - Apply the stoichiometry ratios to calculate the number os moles of Fe that reacted
3 - Use the tomic weight of Fe to calculate the mass of that amount of number of moles of Fe.
1 - The concentration is given by the equation:
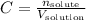
The number of moles of solute is the same as the number of moles o HCl, because it is the solute in this case:
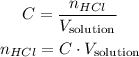
So, we have:
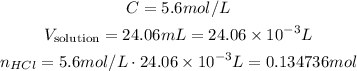
2 - The coefficients of Fe and HCl are 1 and 2, respectively, so we have the following relation between their number of moles:
Fe --- HCl
1 --- 2
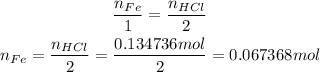
3 - The atomic weight of Fe can be checked on a periodic table:
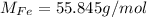
So, we have:
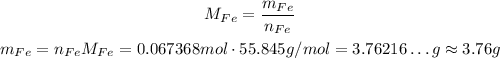
So, there was approximately 3.76 g of Fe.