Step 1 - Balancing the neutralization reaction
Since perchloric acid (HClO4) is an acid and calcium hydroxide (Ca(OH)2) is a base, the reaction will be a neutralization. Note that HClO4 is a monoprotic acid, whereas Ca(OH)2 will liberate, by dissociation, two OH(-) groups. We will need thus two HClO4 (to liberate two H(+) and fully neutralize the OH(-) groups):
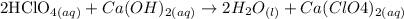
Step 2 - Discovering how many moles of Ca(OH)2 have reacted
Since the exercive gives us the volume (V) as well as the concentration ([ ])of Ca(OH)2, we can discover how many moles (n) of it reacted by using the following relation:
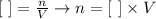
We already know that [Ca(OH)2] = 0.149 mol/L and V = 26 ml (0.026 L). The number of moles will be thus:
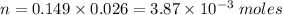
Step 3 - Discovering how many moles of HClO4 have reacted
Looking at the equation in step 1 again:
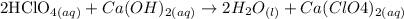
We can see that 2 moles of HClO4 react with 1 mole of Ca(OH)2. This is a fixed proportion which means that we'll always need two times more HClO4 than Ca(OH)2.
The number of moles of HClO4 that reacted will be thus:
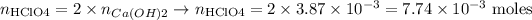
The number of moles of HClO4 that reacted is thus 7.74*10(-3) moles.
Step 4 - Discovering the required volume of HClO4
Now that we know how many moles of HClO4 reacted and given that we also know the concentration (0.224 M), we can discover the volume by using the same formula as in step 2:
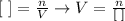
Substituting the values on the equation, we get:
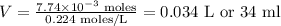
The required volume will be thus 34 ml.