The equilibrium constant relates the concentrations of the products and reactants in the chemical equilibrium. To calculate it we can apply the following equation.
For the reaction: aA + bB ---> cC + dD
Equlibrium constant (Ke) will be:
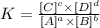
The letters in square brackets refer to the concentration raised to the respective coefficient. Now we replace the known values:
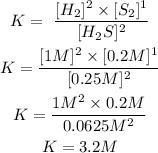
So, the equilibrium constant (Ke) will be 3.2M