Explanation:
For an ideal gas, product of pressure and volume equals n times R times T.
Mathematically, PV = nRT
where P = pressure
V = volume
n = number of moles
R = gas constant
T = temperature
Also, it is known that in 1 kpa there are 0.0098 atm. So, 100.0 kpa equals 0.98 atm. And 275 ml equals 0.275 l. Therefore, calculate temperature as follows.
PV = nRT
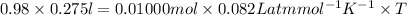
T =
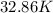
Thus, we can conclude that temperature for the given problem is
 .
.