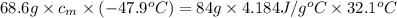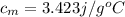Answer: The specific heat of the metal comes out to be
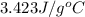
Explanation: Heat absorbed or heat lost, q is written as:
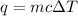
We are given that an unknown metal is dropped in water. So, in this process:
Heat lost by the metal = Heat gained by the water
Mathematically,
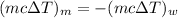 ....(1)
....(1)
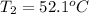
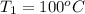
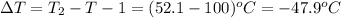
m = 68.6 g
c = ?
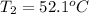
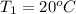
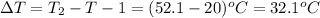
m = 84 g
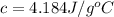
Putting this in equation 1, we get