Answer:
![4NH_3+5O_2\operatorname{\rightarrow}*4NO+6H_2O.]()
10.3 g of NH3 and 24.3 g of O2.
Step-by-step explanation:
First, let's write the unbalanced chemical equation:
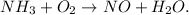
By trial and error method, we can see that the balanced equation would be:
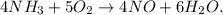
Now that we have the balanced chemical equation, we can find the mass of each reactant required to produce 18.2 g of NO.
We have to convert 18.2 g of NO to moles. The molar mass of NO is 30 g/mol (you can calculate the molar mass using the periodic table). The conversion is:
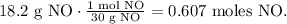
With this value, we can find the moles of NH3 and O2 required. You can see that 4 moles of NH3 reacted produces 4 moles of NO. The molar ratio between these two would be 4:4 and simplifying, it would be 1:1. This means that we need the same number of moles of SO2 for NH3, so the required moles of NH3 are 0.607 moles. Now, let's find its mass by using the molar mass of NH3 which is 17 g/mol:
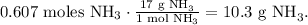
And now, let's find the moles of O2. In the chemical equation, we have 5 moles of O2 reacted to produce 4 moles of NO. So the conversion would look like this:
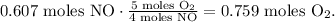
And the final step is to convert 0.759 moles of O2 to grams using the molar mass of O2 which is 32 g/mol:
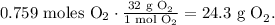
The answer is that we're going to need 10.3 g of NH3 and 24.3 g of O2.