Answer:
B.) The flask on the left is the strong acid because 100% of the molecules dissociate.
Step-by-step explanation:
First, let's review the concept of strong acid: A strong acid is an acid that is completely ionized in an aqueous solution. Let's see an example of this:
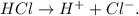
Now, you can see the picture and imagine that the grey circles are Cl and the black circles are H, so as HCl is a strong acid and it dissociates completely in two different ions, the strong acid would be the first flask. It wouldn't be the second flask because there are some molecules that don't dissociate completely (because they are not 'separated').
Based on this logic, the answer would be B.) The flask on the left is the strong acid because 100% of the molecules dissociate.