ANSWER
The volume of Argon gas is 5.60L
Explanation:
Given information:
The number of atoms of argon = 1.5 x 10^23 atoms
To find the volume of Argon, we need to find the number of moles of argon, then, we assume the number of moles of argon to be x
To find the number of moles, we will need to apply the below formula
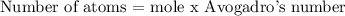
Recall that, Avogadro's number = 6.022 x 10^23
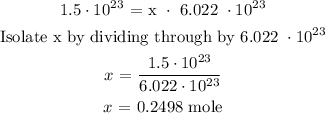
Since x represents the number of moles of argon gas, then, the mole of argon gas is 0.2498 mole.
The next step is to find the volume of Argon in liters
Recall that, at S.T.P, 1 mole is equivalent to 22.4L
Let x represent the volume in liters of argon
Then, this can be solved mathematically below

Therefore, the volume of Argon gas is 5.60L