- First, to calculate the % concentration of glucose in the solution, we need to add the 8.5g of glucose to the 35 g of water, to get the weight of the solution:
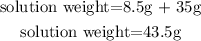
- Second, we calculate the %w/w (weight/weight) with the solute weight and the solution weight:
![undefined]()
- So,