Answer:
The 7.5 L of 5% solution and 2.5 L of 25 % solution .
Explanation:
As given
A chemist currently has two solutions of sodium chloride.
One solution has a 5% concentration and the other has a 25% concentration.
The chemist needs to make 10 L of a 10% sodium chloride solution.
Let x = the amount of 5% solution.
Let y = the amount of 25% solution.
Than first equation becomes
x + y = 10
5% is written in the decimal form .
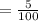
= 0.05
25% is written in the decimal form .
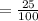
= 0.25
10% is written in the decimal form .
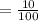
= 0.10
Than the second equations becomes
Concentration of 5% solution × Amount of solution + Concentration of 25% solution × Amount of solution = Concentration of 10% solution × Amount of solution .
Putting all the values in the above
0.05x + 0.25y = 10 × 0.10
Simplify the equation
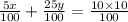
5x + 25y = 100
Than two equations are
x + y = 10
5x + 25y = 100
Multiply x + y = 10 by 5 and subtracted from 5x + 25y = 100 .
5x - 5x + 25y - 5y = 100 - 50
20y = 50
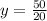
y = 2.5 L
Putting the value of y in the equation
x + y = 10
x + 2.5 = 10
x = 10 - 2.5
x = 7.5 L
Therefore the 7.5 L of 5% solution and 2.5 L of 25 % solution .