Answer : The correct option is, (B) 12.3 KJ
Solution :
First we have to calculate the moles of water.
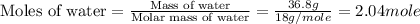
Now we have to calculate the amount of heat released.
Formula used :

where,
q = heat released
n = number of moles = 2.04 mole
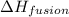 = molar enthalpy of fusion of a substance = -6.01 KJ/mole
= molar enthalpy of fusion of a substance = -6.01 KJ/mole
Now put all the give values in the above formula, we get the amount of heat released.
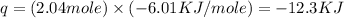
The negative sign indicate heat released.
Therefore, the amount of heat released is, 12.3 KJ