Answer:
11.96 degrees
Explanations
The formula for finding the heat absorbed by the water is expressed as;
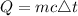
where:
• m is the ,mass, of the substance
,
• c is the ,specific heat ,capacity
,
• △t is the ,change in temperature
Given the following parameters
mass = 100g = 0.1kg
c = 4182 J/kg°C
Q = 5kJ = 5000Joules
Required
Change in temperature
Substitute the given parameters into the formula
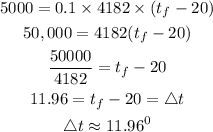
This shows that the temperature of the water will increase by 11.96 degrees