Answer:
17.136 g H2
Step-by-step explanation:
First we need to convert moles of Zinc to moles of Hydrogen in order to find out how many moles of hydrogen are produced.
When we look at our balanced equation, we can see that for every mole of Zn reacting, there is 1 mol of H2 produced. (imagine a 1 in front of the elements that have no numbers in front of them)
8.5 mol Zn *
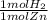 = 8.5 mol H2
= 8.5 mol H2
Now we will convert from moles of Hydrogen to grams
The atomic mass of H2 is 2(1.008) g/1 mol... so we will use this to convert to the number of grams of Hydrogen produced..
8.5 mol H2 *
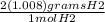 = 17.136 g H2
= 17.136 g H2